Hospitals knew a heart device led to more patients’ deaths

Michael Buchanan,social affairs reporter And
Adam Eley,BBC News
 family statement
family statementDozens of patients have been put at risk after two leading UK transplant centers continued to be fitted with heart devices despite concerns that they had a higher death rate than a rival product.
Concerns about the device were raised by the NHS in 2018. Half of the patients who later received mechanical pumps died within three years.
The mother of Greg Marshall, one of the patients who received the device after his concerns became known and later died, told us she was “disgusted and horrified.”
The BBC has also learned that leading cardiologists at both hospitals acted as paid consultants on behalf of the device maker. Hospitals were also aware of this.
Both hospitals – Freeman in Newcastle and Harefield in London – continued to use the pump for years, questioning the reliability of the data flagged by the NHS. Medtronic, the manufacturer of the pump, eventually withdrew it for safety reasons.
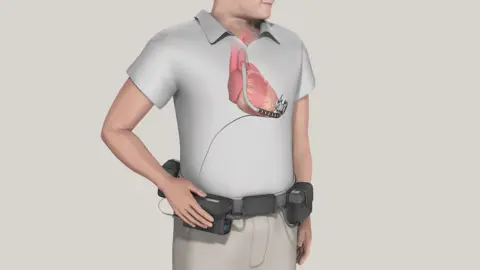
Patients who have a weakened heart, are awaiting a transplant, or are deemed unsuitable for a transplant may be offered a machine called an LVAD, a Left Ventricular Assist Device. It helps the heart pump blood around the body, and for many patients, this is their only chance of survival until they can receive a transplant.
The device is implanted in the heart with an external wire that emerges from the body (usually in the abdomen) and connects to a control unit and external batteries.
LVADs have been lifesaving for decades, and for several years hospitals had a choice of two devices: the HeartWare HVAD, sold by Irish-American company Medtronic, and the Heartmate III, sold by US manufacturer Abbott.
 Medtronic
MedtronicNHS Blood and Transplant (NHSBT), which oversees transplants in the UK, carried out a preliminary audit in October 2018 comparing how the two pumps performed. A more detailed analysis was conducted in April 2019.
The results were striking. Of the 119 patients who received the Medtronic device, 45% (54 patients) died within two years. In contrast, only 15% of 97 patients given the Abbott pump (15 of 97) died during the same period. Similarly, the number of complications, such as stroke or needing a new pump, was significantly higher with the Medtronic device. The audit said there were “no significant differences” between the types of patients who received each device.
The Royal Papworth Hospital in Cambridge, one of England’s six transplant centres, did not wait for the NHS analysis. It had noted growing international concerns and stopped using the Medtronic device in February 2018 “after evaluating the results of two randomized controlled trials” because its clinicians “viewed the Heartmate III as superior.”
However, Harefield Hospital continued to use the Medtronic device exclusively until early 2021, shortly after the manufacturer issued a safety statement. Freeman Hospital continued until June 2021, when the manufacturer pulled it from sale “in the interest of patient safety.”
The regulator, the Medicines and Healthcare products Regulatory Agency (MHRA), went on to approve the use of the device after the 2019 analysis, although it was not informed by the NHS of the existence of the data.
During this period, patients with Medtronic devices continued to die at higher rates than those with Abbott devices.
Figures released under the Freedom of Information Act show that the mortality rate for the Medtronic device was two-and-a-half times higher than that of the Abbott device between October 2018, when the first NHS data was produced, and June 2021, when Medtronic stopped the pump.
Forty-nine percent (39 of 80 patients) of those who received the Medtronic device at that time died within three years. By comparison, 19% of Abbott device recipients (20 of 106 patients) died during the same time period.
 family statement
family statementMedtronic decided to recall the device in June 2021 following growing evidence that its pump had a higher frequency of strokes and deaths compared to other devices. Also addressed was a specific fault where pumps experienced a delay or failed to restart after being stopped.
Greg Marshall was one of the patients implanted with the Medtronic device, unaware of studies on its effectiveness compared to a competing product. A fit and healthy young man, he had dreams of joining the Royal Marines but suffered acute heart failure in 2019.
“It was a huge shock to all of us,” her mother, Tessa Marshall, recalls.
In June of that year, a few months after the first NHS data became known, Freeman Hospital offered Greg the Medtronic device.
Although they brought in a current patient to explain the benefits of the device, their family said they were not faced with any long-term risks of the device.
Greg agreed to surgery, but a major complication at the theater – which the family said was never fully explained to them – led to a stroke. Greg lost movement of the left side of his body and his speech was significantly impaired.
Over time, his health slowly began to improve, but a year later in July 2020, his heart device suddenly stopped working. When I tried to reboot it wouldn’t turn on again.
“The pump wasn’t pumping and was slowly turning blue,” says Tessa.
Greg was taken to the hospital, but the device could not be repaired. He was told he could have the surgery removed, but he refused because he was afraid of having another stroke.
The faulty device remained inside him, turned off, as his heart continued to work for another three years, by which time he was on the transplant waiting list.
In September 2023, he unexpectedly suffered a heart attack while in hospital. By the time Tessa reached Freeman, Greg was dead at the age of 26.
His family is devastated by his loss and says they want answers.
“We said, ‘Look Greg, this is the only option you have, you have to accept it,'” Tessa said. “I’m now angry at myself for not doing more research.”
Newcastle’s NHS Trust told the Department of Health and Social Care in a letter seen by the BBC that three patients may have died as a result of the failure.
The trust has since told the BBC that the issue of the device not restarting in patients first emerged in December 2020 – five months after Greg encountered the problem – when Medtronic issued a safety notice.
 ISHLT
ISHLTHospital documents show Greg’s responsible healthcare professional was Prof Stephan Schueler, who until recently was head of Freeman’s cardiothoracic department. Professor Schueler, described by a former colleague as the “king of the castle” in the transplant unit, had a decade-long relationship with the manufacturer of the Medtronic device, public records show.
Greg’s family said he had not disclosed to them his financial relationship with the heart device manufacturer, despite such disclosure being a requirement of doctors’ regulator, the General Medical Council (GMC).
“There has never been a financial incentive or salary arrangement with Medtronic for me or anyone on our team to choose one device over another,” Prof Schueler told the BBC. He did not respond to a direct question about what he told Greg Marshall about his relationship with Medtronic, but said he “always works within GMC standards for good medical practice” and that patients’ consent for a procedure is “carried out by a team of expert colleagues”.
It is common for clinicians to work as consultants for medical manufacturers and pharmaceutical companies. Such arrangements are considered mutually beneficial and can lead to better outcomes for patients, but there are rules about what healthcare providers must report to patients.
Freeman told the BBC he offered his “sincere condolences” to Greg’s family and said the case was being investigated.
The institution stated that although it was “aware” of the NHS data in April 2019, it felt that its scientific value was not reliable, and pointed out that it was not accepted in any national or international scientific publications. It was said at the time that there were other publications pointing to “excellent results” for the Medtronic device.
NHS Blood and Transplant patient representative Robbie Burns has obtained several documents relating to hospitals’ continued use of the Medtronic device. He believes there should be an external investigation into how this was allowed to happen.
“It was completely preventable,” he said.
“If I were in a position to buy this [Medtronic] When I looked at the device and looked at the data again, I thought, ‘Why did you do this?’ “I thought.”
Prof Schueler said the Medtronic device had many advantages, including being more suitable for children and young adults. He said the decision to continue using the pump was made after internal discussions carefully considered all the risks and benefits.
Another doctor, André Simon, director of heart and lung transplants and ventricular assist devices at Harefield Hospital, had a similarly long-standing relationship with HeartWare and Medtronic, dating back to 2014.
His replacement at the hospital in west London was Dr. John Dunning told the BBC that Harefield continued to use the Medtronic device as his predecessor’s “preference”.
Dr Simon, who no longer works at the hospital, did not answer the BBC’s questions. Harefield said in a statement that he was “aware” of her work for Medtronic, which was disclosed in several documents. The hospital said he was one of several senior people “involved” in choosing which devices to use, adding that “use of the device should continue”. [Medtronic device] It had the collective support of clinicians at Harefield until the manufacturer published a safety statement in early 2021.
In separate statements to the BBC, both Freeman and Harefield hospitals said their decision to continue using the Medtronic device was based on “complex clinical judgments” and stressed that there was “no clear justification” at the time to believe the Medtronic device was significantly inferior to the Abbott device.
Harefield said he commissioned an external review of its services in 2019 from the then chair of NHSBT’s Cardiothoracic Advisory Group, which made no comment on the choice of device.





